Medical Device
DV7 Engineers have been providing complex medical device product and software development solutions for many world-leading medical device companies for many years. Few teams can match the level of safety-critical expertise of DV7. Whether you are adding functionality to existing equipment. Need Independent Verification and Validation, have a napkin and an idea and, need it brought through the process. DV7 will ensure it is designed, documented, and tested in the proper fashion for the certification required. This kind of technologically advanced product needs a team that has been there and done that. That is DV7 Engineering.
Product Development
- DV7 Engineering is highly experienced, focused, from napkin to prototype the right way the DV7 way!
- Experience in embedded System and Sub-System Development
- Industry knowledge of medical device engineering, usability, and feasibility
- Equipped to handle electro-mechanical and software development
- Built in INDEPENDENT V&V team. All testing can be legally and properly completed and documented under one roof.
- Solutions geared for FDA/CE/ MDR approvals
- Adhere to ISO 13485 and standards such as IEC 60601, IEC 62304, to name a few.
- Virtually unlimited capabilities to prototype, design, and design transfer to manufacturing
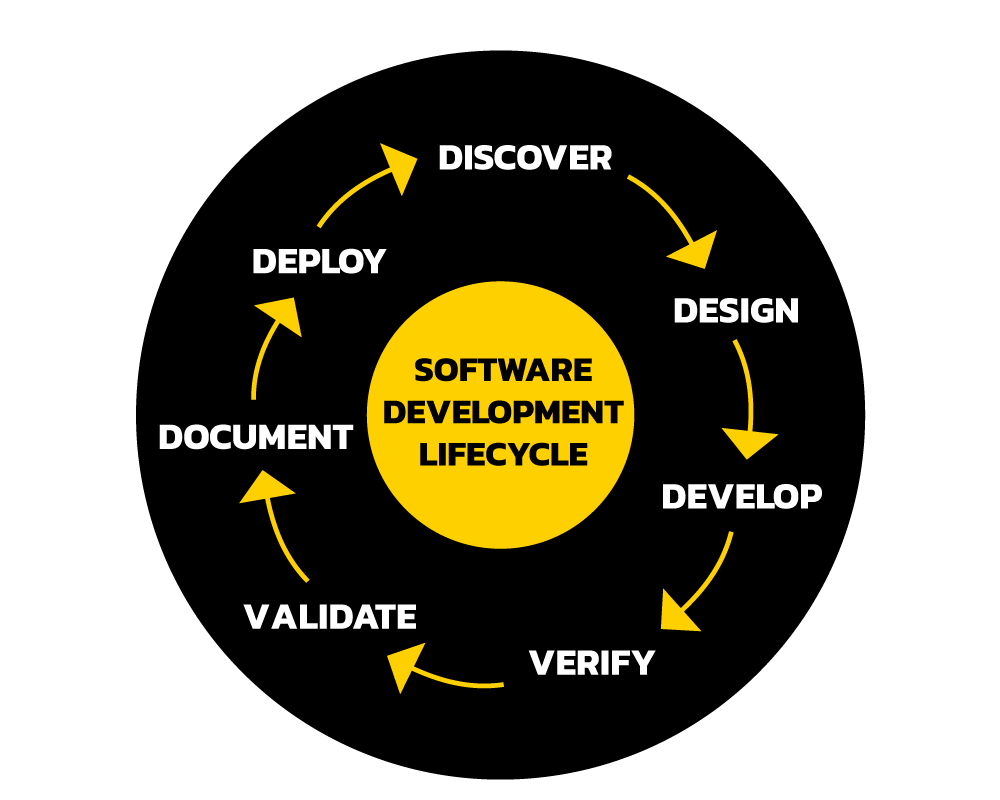
Software Development
Medical device software development at DV7 follows ISO 13485:2016 standards and adheres to IEC and FDA/ federal regulations, such as, 14971, 60601, 62304, HE75, CFR 820.30, and HIPAA. This is all built into the DV7 Quality Management System (QMS). We understand that not all medical software products have the same level of regulatory requirements, risk and criticality. We develop a customized approach that best serves the requirements of Class I, Class II, and Class III device services. This can affect areas such as the testing and documentation required by the level of product being developed. This will also greatly affect time to market, and cost. Choosing DV7 will ensure you do not overcomplicate the process, add time and costs. We will do what is best practice for the safest and the best software product possible.
IV&V Independent Verification & Validation
IV&V- Independent Verification & Validation is an engineering process employing rigorous methodologies, and the use of tools for evaluating the correctness and quality of the software product throughout the software lifecycle. Software IV&V is adapted to the characteristics of the project, and the level of risk and criticality.
IV&V the DV7 way;
- Includes risk identification and mitigation techniques
- Provides independent evaluation and assessment of:
- Are we building the product correctly? Which is verification
- Did we build the correct product? Which is validation
- Requires technical, managerial, and financial independence see figure 1.
DV7 Way vs. the Wrong Way of designing and implementing a medical device can eliminate technical debt which can end up costing 10X-100X.
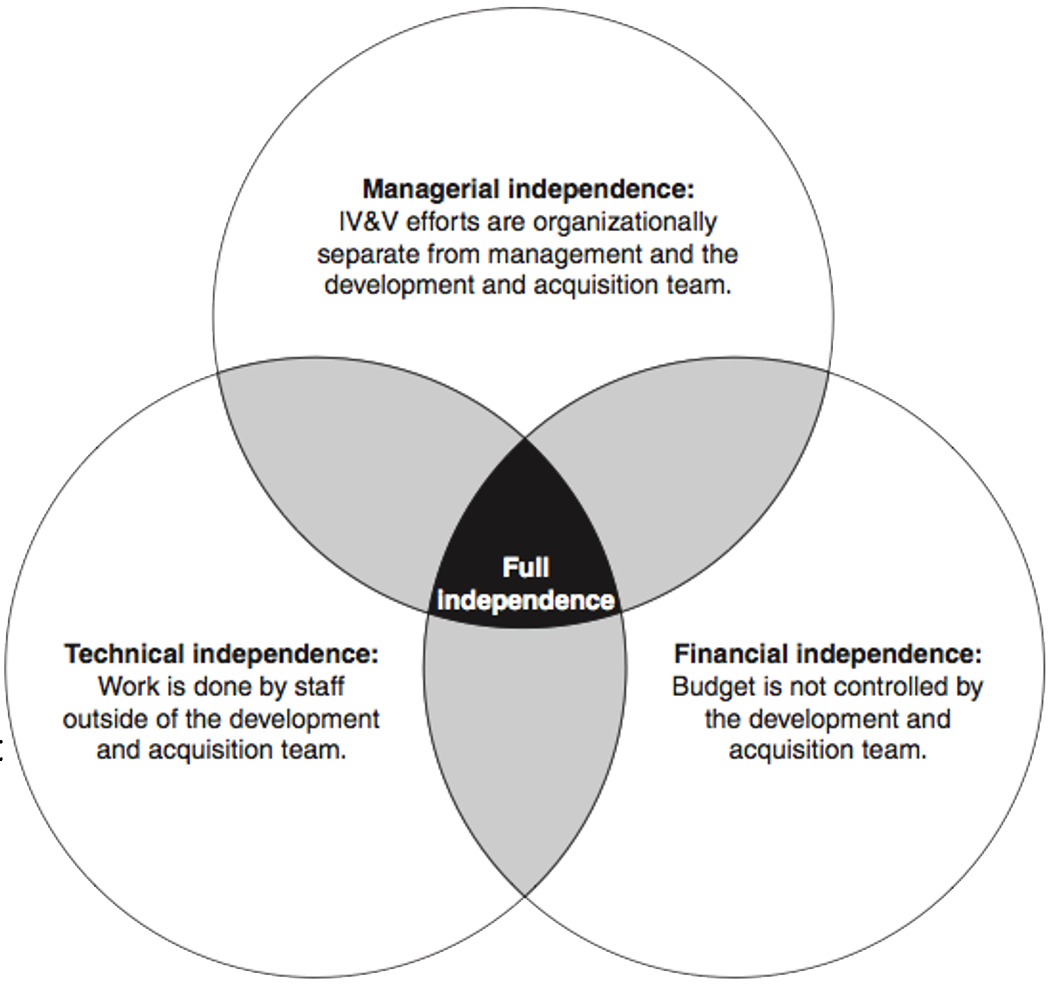
Figure 1
DV7 Engineering
55 Hartland Street, Suite 403, East Hartford, CT 06108
team@dv7engineering.com
860 969 1960
Stay Connected
Want to know what DV7 is up to? Follow us on your favorite platform.
Committed to our Customers
DV7 Engineering is committed to the success of our clients. We strive to educate, collaborate, and partner on projects to solve problems and create superior products.
Let's Get Started!
Our team of engineers are ready for a challenge. We would love to learn and collorboate on your next project. Fill out the form below and let's get started!
